Description
Grazoprevir, also known as MK5172, is a drug approved for the treatment of hepatitis C. Grazoprevir is a second generation hepatitis C virus protease inhibitor acting at the NS3/4a protease targets. It has good activity against a range of HCV genotype variants, including some that are resistant to most currently used antiviral medications.
Product information
CAS Number: 1350462-55-3
Molecular Weight: 784.92
Formula: C38H52N6O10S
Synonym:
Grazoprevir hydrate
MK5172 hydrate
MK 5172 hydrate
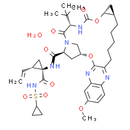
Chemical Name: (1R,18R,20R,24S,27S)-24-tert-butyl-N-[(1R,2S)-1-[(cyclopropanesulfonyl)carbamoyl]-2-ethenylcyclopropyl]-7-methoxy-22,25-dioxo-2,21-dioxa-4,11,23,26-tetraazapentacyclo[24.2.1.0³,¹².0⁵,¹⁰.0¹⁸,²⁰]nonacosa-3,5(10),6,8,11-pentaene-27-carboxamide hydrate
Smiles: O.CC(C)(C)[C@@H]1NC(=O)O[C@@H]2C[C@H]2CCCCCC2=NC3=CC=C(C=C3N=C2O[C@@H]2C[C@@H](C(=O)N[C@@]3(C[C@H]3C=C)C(=O)NS(=O)(=O)C3CC3)N(C2)C1=O)OC
InChiKey: RXSARIJMSJWJLZ-CIAYNJNFSA-N
InChi: InChI=1S/C38H50N6O9S.H2O/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27;/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47);1H2/t21-,22-,24-,29+,30-,31-,38-;/m1./s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
References:
- Yeh WW, Fraser IP, Jumes P, Petry A, Lepeleire I, Robberechts M, Reitmann C, Van Dyck K, Huang X, Guo Z, Panebianco D, Nachbar RB, O'Mara E, Wagner JA, Butterton JR, Dutko FJ, Moiseev V, Kobalava Z, Hüser A, Visan S, Schwabe C, Gane E, Popa S, Ghicavii N, Uhle M, Wagner F. Antiviral Activity, Safety, and Tolerability of Multiple Ascending Doses of Elbasvir or Grazoprevir in Participants Infected With Hepatitis C Virus Genotype-1 or -3. Clin Ther. 2018 Apr 24. pii: S0149-2918(18)30094-8.
- Zamor PJ, Vierling J, Ghalib R, Luketic V, Ravendhran N, Balart L, Robertson M, Hwang P, Hanna GJ, Nguyen BY, Barr E, Talwani R, Pearlman B. Elbasvir/grazoprevir in black adults with hepatitis C virus infection: a pooled analysis of phase 2/3 clinical trials. Am J Gastroenterol. 2018 Apr 26.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.