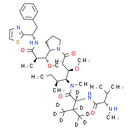
Description
D8-MMAD is a deuterated form of MMAD, which is a microtubule disrupting agent.
Product information
Molecular Weight: 779.11
Formula: C41H66N6O6S
Synonym:
Demethyldolastatin 10 D8
Monomethylauristatin D D8
Monomethyl Dolastatin 10 D8
Chemical Name: (2S)-N-[(3R, 4S, 5S)-3-methoxy-1-[(2S)-2-[(1R, 2R)-1-methoxy-2-methyl-2-{[(1S)-2-phenyl-1-(1, 3-thiazol-2-yl)ethyl]carbamoyl}ethyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-3-(H)methyl-N-methyl-2-[(2S)-3-methyl-2-(methylamino)butanamido](H)butanamide
Smiles: [2H]C([C@]([2H])(NC(=O)[C@@H](NC)C(C)C)C(=O)N(C)[C@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@@H](CC1C=CC=CC=1)C1=NC=CS1)OC)[C@@H](C)CC)(C([2H])([2H])[2H])C([2H])([2H])[2H]
InChiKey: BLUGYPPOFIHFJS-JVNODLFUSA-N
InChi: InChI=1S/C41H66N6O6S/c1-12-27(6)36(46(9)41(51)35(26(4)5)45-39(50)34(42-8)25(2)3)32(52-10)24-33(48)47-21-16-19-31(47)37(53-11)28(7)38(49)44-30(40-43-20-22-54-40)23-29-17-14-13-15-18-29/h13-15,17-18,20,22,25-28,30-32,34-37,42H,12,16,19,21,23-24H2,1-11H3,(H,44,49)(H,45,50)/t27-,28+,30-,31-,32+,34-,35-,36-,37+/m0/s1/i4D3,5D3,26D,35D
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO : ≥ 100 mg/mL (128.35 mM)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥360 days if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
MMAD (Monomethyl Dolastatin 10) is coupled through a stable oxime-ligation process to yield several near-homogenous antibody-drug conjugates (ADCs) with a drug-to-antibody ratio of ~2.0. The resulting conjugates demonstrate good pharmacokinetic properties, potent in vitro cytotoxic activity against HER2+ cancer cells. When compared with ADCs prepared by cysteine alkylation following native interchain disulfide reduction, site-specific unnatural-amino-acid-based ADCs are shown to have increased in vitro cytotoxicity.
In Vivo:
The resulting antibody-drug conjugates (ADCs) demonstrate complete tumour regression in rodents. They also have an improved toxicology profile in rats.
References:
- Chudasama V, et al. Recent advances in the construction of antibody-drug conjugates. Nat Chem. 2016 Feb;8(2):114-9.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.