Description
Benzophenonetetracarboxylic acid (3,3',4,4'-Benzophenonetetracarboxylic acid) is particularly useful in the preparation of high performance polyimides and also useful as curing agents for epoxy resins.
Product information
CAS Number: 2479-49-4
Molecular Weight: 358.26
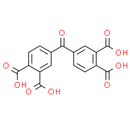
Formula: C17H10O9
Chemical Name: 4-(3,4-dicarboxybenzoyl)benzene-1,2-dicarboxylic acid
Smiles: OC(=O)C1=CC=C(C=C1C(O)=O)C(=O)C1=CC(=C(C=C1)C(O)=O)C(O)=O
InChiKey: UITKHKNFVCYWNG-UHFFFAOYSA-N
InChi: InChI=1S/C17H10O9/c18-13(7-1-3-9(14(19)20)11(5-7)16(23)24)8-2-4-10(15(21)22)12(6-8)17(25)26/h1-6H,(H,19,20)(H,21,22)(H,23,24)(H,25,26)
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO : 50 mg/mL (139.56 mM; Need ultrasonic). H2O : 10 mg/mL (27.91 mM; Need ultrasonic).
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
The kinetics of the photooxidation of aromatic amino acids histidine (His), tyrosine (Tyr), and tryptophan (Trp) by Benzophenonetetracarboxylic acid has been investigated in aqueous solutions using time-resolved laser flash photolysis and time-resolved chemically induced dynamic nuclear polarization. The pH dependence of quenching rate constants is measured within a large pH range. The chemical reactivities of free His, Trp, and Tyr and of their acetylated derivatives, N-AcHis, N-AcTyr, and N-AcTrp, toward Benzophenonetetracarboxylic acid triplets are compared to reveal the influence of amino group charge on the oxidation of aromatic amino acids. Thus, it has been established that the presence of charged amino group changes oxidation rates by a significant factor; i.e., His with a positively charged amino group quenches the Benzophenonetetracarboxylic acid triplets 5 times more effectively than N-AcHis and His with a neutral amino group. The efficiency of quenching reaction between the Benzophenonetetracarboxylic acid triplets and Tyr and Trp with a positively charged amino group is about 3 times as high as that of both Tyr and Trp with a neutral amino group, N-AcTyr and N-AcTrp.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.