Description
Rifamycin S, a quinone and an antibiotic against Gram-positive bacteria (including MRSA), is a clinical drug used to treat tuberculosis and leprosy. Rifamycin S generates reactive oxygen species (ROS) and inhibits microsomal lipid peroxidation.
Product information
CAS Number: 13553-79-2
Molecular Weight: 695.75
Formula: C37H45NO12
Chemical Name: (7S, 11S, 12R, 13S, 14R, 15R, 16R, 17S, 18S)-2, 15, 17-trihydroxy-11-methoxy-3, 7, 12, 14, 16, 18, 22-heptamethyl-6, 23, 27, 29-tetraoxo-8, 30-dioxa-24-azatetracyclo[23.3.1.1, .0, ]triaconta-1, 3, 5(28), 9, 19, 21, 25-heptaen-13-yl acetate
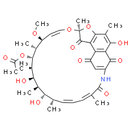
Smiles: CO[C@H]1C=CO[C@@]2(C)OC3=C(C4=C(C(=O)C(=CC4=O)NC(=O)C(C)=CC=C[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)C(O)=C3C)C2=O
InChiKey: BTVYFIMKUHNOBZ-WURVRSKISA-N
InChi: InChI=1S/C37H45NO12/c1-16-11-10-12-17(2)36(46)38-23-15-24(40)26-27(32(23)44)31(43)21(6)34-28(26)35(45)37(8,50-34)48-14-13-25(47-9)18(3)33(49-22(7)39)20(5)30(42)19(4)29(16)41/h10-16,18-20,25,29-30,33,41-43H,1-9H3,(H,38,46)/b11-10-,14-13-,17-12-/t16-,18+,19+,20+,25-,29-,30+,33+,37-/m0/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Solubility (25°C). 100 mg/mL(143.72 mM). Insoluble.
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
The inhibition of bacterial growth by Rifamycin SV is due to the production of active species of oxygen resulting from the oxidation-reduction cycle of Rifamycin SV in the cells. The aerobic oxidation of Rifamycin SV to Rifamycin S is induced by metal ions, such as Mn2+, Cu2+, and Co2+. The most effective metal ion is Mn2+.
In Vivo:
Rat liver sub-mitochondrial particles also generated hydroxyl radical in the presence of NADH and Rifamycin S. NADH dehydrogenase (complex I) as the major component involved in the reduction of Rifamycin S. Compared to NADPH, NADH is almost as effective (Rifamycin S) in catalyzing the interactions of these antibiotics with rat liver microsomes. Rifamycin S is shown to be readily reduced to Rifamycin SV, the corresponding hydroquinone by Fe(II). Rifamycin S forms a detectable Fe(II)-(Rifamycin S)3 complex. The Fe:ATP induced lipid peroxidation is completely inhibited by Rifamycin S. Rifamycin S can interact with rat liver microsomes to undergo redox-cycling, with the subsequent production of hydroxyl radicals when iron complexes are present.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.