Description
(S,R,S)-AHPC-C8-NH2 dihydrochloride (VH032-C8-NH2 dihydrochloride) is a synthesized E3 ligase ligand-linker conjugate that incorporates the VH032 based VHL ligand and a linker used for AKT PROTAC degrader. (S,R,S)-AHPC-C8-NH2 is XF038-164A, example 8, extracted from patent WO2019173516A1.
Product information
CAS Number: 2341796-80-1
Molecular Weight: 658.72
Formula: C31H49Cl2N5O4S
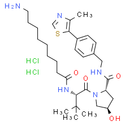
Chemical Name: (2S,4R)-1-[(2S)-2-(9-aminononanamido)-3,3-dimethylbutanoyl]-4-hydroxy-N-{[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}pyrrolidine-2-carboxamide; bis(chlorohydrogen)
Smiles: Cl.Cl.CC1N=CSC=1C1=CC=C(CNC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)CCCCCCCCN)C(C)(C)C)C=C1
InChiKey: RBLHRVOQDCWDGC-OXLNOHKBSA-N
InChi: InChI=1S/C31H47N5O4S.2ClH/c1-21-27(41-20-34-21)23-14-12-22(13-15-23)18-33-29(39)25-17-24(37)19-36(25)30(40)28(31(2,3)4)35-26(38)11-9-7-5-6-8-10-16-32;;/h12-15,20,24-25,28,37H,5-11,16-19,32H2,1-4H3,(H,33,39)(H,35,38);2*1H/t24-,25+,28-;;/m1../s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
References:
- Chan KH, et al. Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds. J Med Chem. 2018 Jan 25;61(2):504-513.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.