Description
Polymyxin B nonapeptide is a cyclic peptide obtained from Polymyxin B by proteolytic removal of its terminal amino acyl residue. Polymyxin B nonapeptide is less toxic, lacks bactericidal activity, and retains its ability to render gram-negative bacteria susceptible to several antibiotics by permeabilizing their outer membranes.
Product information
CAS Number: 86408-36-8
Molecular Weight: 963.13
Formula: C43H74N14O11
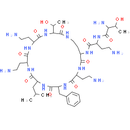
Chemical Name: (2S, 3R)-2-amino-N-[(2S)-4-amino-1-oxo-1-[[(3S, 6S, 9S, 12S, 15R, 18S, 21S)-6, 9, 18-tris(2-aminoethyl)-15-benzyl-3-[(1R)-1-hydroxyethyl]-12-(2-methylpropyl)-2, 5, 8, 11, 14, 17, 20-heptaoxo-1, 4, 7, 10, 13, 16, 19-heptazacyclotricos-21-yl]amino]butan-2-yl]-3-hydroxybutanamide
Smiles: CC(C)CC1NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CCN)NC(=O)C(CCNC(=O)C(NC(=O)C(CCN)NC(=O)C(CCN)NC1=O)C(C)O)NC(=O)C(CCN)NC(=O)C(N)C(C)O
InChiKey: PYHYGIPVYYRJHU-UHFFFAOYSA-N
InChi: InChI=1S/C43H74N14O11/c1-22(2)20-31-40(65)52-26(10-15-44)35(60)51-29(13-18-47)39(64)57-34(24(4)59)43(68)49-19-14-30(53-36(61)28(12-17-46)54-42(67)33(48)23(3)58)38(63)50-27(11-16-45)37(62)56-32(41(66)55-31)21-25-8-6-5-7-9-25/h5-9,22-24,26-34,58-59H,10-21,44-48H2,1-4H3,(H,49,68)(H,50,63)(H,51,60)(H,52,65)(H,53,61)(H,54,67)(H,55,66)(H,56,62)(H,57,64)
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO : 16.67 mg/mL (17.31 mM; Need ultrasonic). H2O : ≥ 100 mg/mL (103.83 mM).
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Polymyxin B nonapeptide, a cationic cyclic peptide derived by enzymatic processing from the naturally occurring peptide polymyxin B, is able to increase the permeability of the outer membrane of Gram-negative bacteria toward hydrophobic antibiotics probably by binding to the bacterial lipopolysaccharide (LPS).
References:
- Tsubery H, et al. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of gram-negative bacteria. J Med Chem. 2000 Aug 10;43(16):3085-92.
- Ofek I, et al. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob Agents Chemother. 1994 Feb;38(2):374-7.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.