Description
Dexamethasone is a type of steroid medication. It has anti-inflammatory and immunosuppressant effects. It is 25 times more potent than cortisol in its glucocorticoid effect, while having minimal mineralocorticoid effect. Dexamethasone is used for the treatment of many conditions including: rheumatologic problems, a number of skin diseases such as erythema multiforme, severe allergies, asthma, chronic obstructive lung disease, croup, and cerebral edema, in addition to other medications in tuberculosis and a number of other infectious diseases.
Product information
CAS Number: 50-02-2
Molecular Weight: 392.46
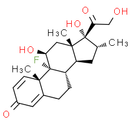
Formula: C22H29FO5
Synonym:
Decaject
Decaject L.A.
Decaject-L.A.
Decameth
Decaspray
Dexasone
Dexpak
Hexadecadrol
Hexadrol
Maxidex
Methylfluorprednisolone
Millicorten
Oradexon
Related CAS Number:
3936-02-5 (Dexamethasone metasulfobenzoate sodium)
3800-84-8 (Dexamethasone sodium succinate)
1177-87-3 (Dexamethasone acetate)
150587-07-8 (Dexamethasone Beloxil)
132245-57-9 (Dexamethasone cipecilate)
2265-64-7 (Dexamethasone isonicotinate)
14899-36-6 (Dexamethasone palmitate)
312-93-6 (Dexamethasone phosphate)
Chemical Name: (8S, 9R, 10S, 11S, 13S, 14S, 16R, 17R)-9-fluoro-11, 17-dihydroxy-17-(2-hydroxyacetyl)-10, 13, 16-trimethyl-6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one
Smiles: C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)CO
InChiKey: UREBDLICKHMUKA-CXSFZGCWSA-N
InChi: InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO 78 mg/mL (198.75 mM) Ethanol 11 mg/mL (28.03 mM)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Dexamethasone results in decrease in transmonolayer paracellular permeability mainly to sucrose, fluorescein and dextrans of up to 20 KDa in an immortalised rat brain endothelial cell line (GPNT). Dexamethasone results in filamentous actin and the cytoskeleton associated protein cortactin being highly concentrated in the regions of cell-cell contact with few F-actin stress fibres visible within the cytoplasm in cultured rat brain endothelial cells, an observation consistent with a more differentiated barrier phenotype induced by dexamethasone. Dexamethasone treatment has been shown to strongly stimulate the level of the Id-1 protein, which is a serum-inducible helix-loop-helix transcriptional repressor, involved in cell differentiation, and this effect was shown to be associated with reorganisation of ZO-1 to the cell periphery in Con8 mammary epithelial tumor cells. Dexamethasone prevents cytokine-induced enhanced expression of MMP-9 and alterations in the expression of ZO-1 in untreated GPNT monolayers. Dexamethasone depletes both basal and TNF-alpha-stimulated GSH levels by down-regulating the gamma-GCS-heavy subunit transcription via a mechanism involving AP-1 (c-Jun) in alveolar epithelial cells. Dexamethasone decreases both basal and stimulated GSH levels (TNF-α-treated) in alveolar epithelial cells (A549), without any change in GSSG.
In Vivo:
Dexamethasone is administered i.m. to pregnant ewes, leads to the following results (1) blood pressure is unchanged; (2) as previously reported in the fetus, sensitivity to endothelin-1 (ET) is increased; (3) acetylcholine-induced relaxation is increased; (4) L-NAME suppressible vasodilatory response to ET is abolished; (5) there is no change in endothelium-independent vasodilatation; and (6) there is no change in eNOS RNA and protein levels, when compared to saline treated controls.
References:
- Romero IA, et al. Neurosci Lett, 2003, 344(2), 112-116.
- Rahman I, et al. Biochem Pharmacol, 2000, 60(8), 1041-1049.
- Molnar J, et al. J Physiol, 2003, 547(Pt 1), 61-66.
- Suzuki E, et al. Clin Cancer Res, 2005, 11(18), 6713-6721.
- Kunnumakkara AB, et al. Cancer Res, 2007, 67(8), 3853-3861.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.