Description
GSK256066 is a potent and selective PDE4 inhibitor that can be given by inhalation, minimising the potential for side effects. GSK256066 demonstrated a protective effect on the EAR and LAR. GSK256066 is a slow and tight binding inhibitor of PDE4B (apparent IC(50) 3.2 pM; steady-state IC(50) <0.5 pM), which is more potent than any previously documented compound, for example, roflumilast (IC(50) 390 pM), tofimilast (IC(50) 1.6 nM), and cilomilast (IC(50) 74 nM). Consistent with this, GSK256066 inhibited tumor necrosis factor α production by lipopolysaccharide (LPS)-stimulated human peripheral blood monocytes with 0.01 nM IC(50). GSK256066 has been demonstrated to have exceptional potency in vitro and in vivo and is being clinically investigated as a treatment for chronic obstructive pulmonary disease.
Product information
CAS Number: 801312-28-7
Molecular Weight: 518.58
Formula: C27H26N4O5S
Synonym:
GSK256066
GSK-256066
GSK 256066
Related CAS Number:
801315-14-0 (HCl)
1415560-64-3 (TFA)
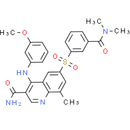
Chemical Name: 6-[3-(dimethylcarbamoyl)benzenesulfonyl]-4-[(3-methoxyphenyl)amino]-8-methylquinoline-3-carboxamide
Smiles: CC1=CC(=CC2=C1N=CC(C(N)=O)=C2NC1=CC(=CC=C1)OC)S(=O)(=O)C1=CC(=CC=C1)C(=O)N(C)C
InChiKey: JFHROPTYMMSOLG-UHFFFAOYSA-N
InChi: InChI=1S/C27H26N4O5S/c1-16-11-21(37(34,35)20-10-5-7-17(12-20)27(33)31(2)3)14-22-24(16)29-15-23(26(28)32)25(22)30-18-8-6-9-19(13-18)36-4/h5-15H,1-4H3,(H2,28,32)(H,29,30)
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Solubility (25°C) DMSO: 5 mg/mL(9.64 mM). Water: Insoluble.
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
GSK256066 is a slow and tight binding inhibitor of PDE4B with apparent IC50 of 3.2 pM. GSK256066 is an extremely potent inhibitor of LPS-stimulated TNFα production in PBMCs with pIC50 of 11.0 and IC50 of 10 pM and human whole-blood cultures with pIC50 of 9.90 and IC50 of 126 pM. GSK256066 is highly selective for PDE4 (>3.8 × 105-fold versus PDE1, PDE2, PDE3, PDE5, and PDE6 and >2.5 × 103-fold against PDE7). GSK256066 inhibits PDE4 isoforms A-D with equal affinity.
In Vivo:
GSK256066 inhibits the LPS-induced pulmonary neutrophilia with an ED50 of 1.1 μg/kg, achieving maximal inhibition of 72% at 30 μg/kg when given in the aqueous suspension. GSK256066 inhibits the LPS-induced pulmonary neutrophilia with ED50 of 2.9 μg/kg, achieving maximal inhibition of 62% when given in the dry powder formulation. GSK256066 shows a moderate plasma clearance of 39 ml/min/kg, a moderate volume of distribution of 0.8 L/kg, and a relatively short half-life of 1.1 hour in the male CD rat. GSK256066 sustains at a high lung concentration of 2.6 μg/g after intra-tracheal administration as an aqueous suspension at a dose of 30 μg/kg in rats. GSK256066 (10 μg/kg) is administered intratracheally at different times (2, 6, 12, 18, 24, and 36 hours) before LPS administration, inhibiting LPS-Induced Pulmonary Neutrophilia in rat lipopolysaccharide (LPS)-induced models of acute pulmonary inflammation. GSK256066 (0.3–100 μg/kg) inhibits LPS-induced increases in exhaled nitric oxide with ED50 of 35 μg/kg in rat. GSK256066 (10 μg/kg) is administered half a hour before OVA administration in rat, inhibiting OVA-induced pulmonary eosinophilia with ED50 of 0.4 μg/kg. GSK256066 administered intratracheally as a dry powder blended in respiratory-grade lactose at doses of 3 to 100 μg/kg 2 hours before inhaled LPS challenge in ferrets, inhibiting LPS-induced pulmonary neutrophilia with ED50 of 18 μg/kg without inducing emetic episodes.
References:
- Nials AT, et al. J Pharmacol Exp Ther, 2011, 337(1), 137-144.
- Woodrow MD, et al. Bioorg Med Chem Lett, 2009, 19(17), 5261-5265.
- Tralau-Stewart CJ, et al. J Pharmacol Exp Ther, 2011, 337(1), 145-154.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.