Description
BETP, CAS No. 1371569-69-5, is positive allosteric modulator and partial agonist of the glucagon-like peptide 1 (GLP-1) receptor. It covalently modifies cysteines 347 and 438 in GLP-1R. Specificity studies have shown that it has no activity on GLP-2, GIP, PTH or glucagon receptors. BETP has been shown to potentiate GLP-1R–dependent intracellular calcium mobilization but not cAMP accumulation in response to GLP-1(7-36)NH2 in recombinant cell lines. Conversely, BETP can enhance cAMP efficacy of GLP-1(9-36)NH2 at GLP-1R but not intracellular calcium mobilization. BETP also potentiates cAMP production of the dual-acting GLP-1R/glucagon (GCG) receptor (GCGR) agonist oxyntomodulin at GLP-1R. It promotes GLP-1(9-36)NH2–mediated glucose-dependent insulin secretion in rodent and human islet preparations as well as in rodent models following intravenous administration.
Product information
CAS Number: 1371569-69-5
Molecular Weight: 406.42
Formula: C20H17F3N2O2S
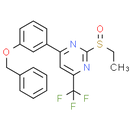
Chemical Name: 4-(3-(benzyloxy)phenyl)-2-(ethylsulfinyl)-6-(trifluoromethyl)pyrimidine
Smiles: CCS(=O)C1N=C(C=C(N=1)C1=CC(=CC=C1)OCC1C=CC=CC=1)C(F)(F)F
InChiKey: NTDFYGSSDDMNHI-UHFFFAOYSA-N
InChi: InChI=1S/C20H17F3N2O2S/c1-2-28(26)19-24-17(12-18(25-19)20(21,22)23)15-9-6-10-16(11-15)27-13-14-7-4-3-5-8-14/h3-12H,2,13H2,1H3
Technical Data
Appearance: Solid Power.
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO up to 100 mM
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
BETP was used at 5-20 µM in vitro and cellular assays.
In Vivo:
BETP was administered through intravenous administration at 5 mg/kg to Wistar rats for IVGTT (In Vivo Intravenous Glucose Tolerance Test). Formulation: 10% ethanol-Solutol, 20% polyethylene glycol-400, and 70% phosphate-buffered saline, pH 7.4.
References:
- Sloop KW, et al. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. (2010) Diabetes. 59(12):3099-107.
- Cheong YH, et al. Two small molecule agonists of glucagon-like peptide-1 receptor modulate the receptor activation response differently. (2012) Biochem Biophys Res Commun. 417(1):558-63.
- Willard FS, et al. Small molecule allosteric modulation of the glucagon-like Peptide-1 receptor enhances the insulinotropic effect of oxyntomodulin. (2012) Mol Pharmacol. 82(6):1066-73.
Products are for research use only. Not for human use.
Documents
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.