Description
Anacetrapib, also known as MK-0859, CAS No. 875446-37-0, is a CETP inhibitor being developed to treat hypercholesterolemia (elevated cholesterol levels) and prevent cardiovascular disease.
Product information
CAS Number: 875446-37-0
Molecular Weight: 637.51
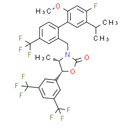
Formula: C30H25F10NO3
Synonym:
MK0859
Anacetrapib
MK-0859
MK 0859
Chemical Name: (4S,5R)-5-(3,5-bis(trifluoromethyl)phenyl)-3-((4'-fluoro-5'-isopropyl-2'-methoxy-4-(trifluoromethyl)-[1,1'-biphenyl]-2-yl)methyl)-4-methyloxazolidin-2-one.
Smiles: CC(C)C1=CC(=C(C=C1F)OC)C1=CC=C(C=C1CN1[C@@H](C)[C@H](OC1=O)C1C=C(C=C(C=1)C(F)(F)F)C(F)(F)F)C(F)(F)F
InChiKey: MZZLGJHLQGUVPN-HAWMADMCSA-N
InChi: InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO, not in water
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
Anacetrapib dose-dependently and significantly decreases the transfer of CE from HDL3 to HDL2 (P<0.001 for concentrations equal to and higher than 0.1 µM). Excess Anacetrapib (25 µM) decreases the amount of [14C]Torcetrapib (0.25 µM) binds to immobilized rhCETP by 82% and 60%, respectively. Anacetrapib decreases pre-β-HDL formation by more than 46% (P<0.001) at all concentrations tested (0.1, 1, 3, and 10 µM). A significant reduction of PCSK9 promoter activity by Anacetrapib (ANA) is detected at 3 µM concentration (−22%, p<0.01) and further lowered to 68% of control at 10 µM. Likewise, luciferase activity of B11 cells are decreased by Anacetrapib at 3 µM concentration and reached to a maximal reduction of 38% of control at 10 µM. At 10 µM concentration, Anacetrapib loweres PCSK9 mRNA level to 60% of control and LDLR mRNA level to 67% of control.
In Vivo:
Hamsters are given Anacetrapib for 7 days before injection of [3H]cholesterol-labeled macrophages (day 0). Treatment with Anacetrapib leads to significant increases in HDL-C levels at day 0. At day 3, [3H]cholesterol radioactivity in the HDL fraction is significantly increased from control values for Anacetrapib. Anacetrapib (ANA) treatment modestly elevates serum total serum cholesterol levels ~10% (p<0.05) and increases serum LDL-C by 26% (p<0.05) as compared to vehicle control. After an intravenous dose of 0.5 mg/kg, the mean values for systemic plasma clearance, steady-state volume of distribution, and terminal half-life are 2.3 mL/min/kg, 1.1 L/kg, and 12 h, respectively. After oral dosing at 5 mg/kg, the bioavailability of Anacetrapib is 38%. Exposures (AUC) increases in a less than dose-proportional manner from 23 μM•h at 5 mg/kg to 362 μM•h at 500 mg/kg. In this dose range, the peak plasma level (Cmax) ranges from 5 to 26 μM and the time to reach peak plasma level (Tmax) ranged from 3 to 4.5 h.
References:
- Krishna R, Stypinski D, Ali M, Garg A, Cote J, Maes A, Degroot B, Liu Y, Li S, Connolly SM, Wagner JA, Stoch SA. Lack of a meaningful effect of anacetrapib on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2012 Jul;74(1):116-24. doi: 10.1111/j.1365-2125.2012.04171.x. PubMed PMID: 22243494.
- Hooper AJ, Burnett JR. Anacetrapib, a cholesteryl ester transfer protein inhibitor. Expert Opin Investig Drugs. 2012 Jan;21(1):103-9. Epub 2011 Dec 22. PubMed PMID: 22191425.
- Krauss RM, Wojnooski K, Orr J, Geaney JC, Pinto CA, Liu Y, Wagner JA, Luk JM, Johnson-Levonas AO, Anderson MS, Dansky HM. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012 Mar;53(3):540-7. Epub 2011 Dec 17. PubMed PMID: 22180633; PubMed Central PMCID: PMC3276477.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.