Description
BGJ-398, also known as, Infigratinib or NVP-BGJ398, CAS No. 872511-34-7, is a pan FGFR kinase inhibitor, and is an orally bioavailable pan inhibitor of human fibroblast growth factor receptors (FGFRs) with potential antiangiogenic and antineoplastic activities. pan FGFR kinase inhibitor BGJ398 selectively binds to and inhibits the activities of FGFRs, which may result in the inhibition of tumor angiogenesis and tumor cell proliferation, and the induction of tumor cell death.
Product information
CAS Number: 872511-34-7
Molecular Weight: 560.48
Formula: C26H31Cl2N7O3
Synonym:
BGJ398
BGJ-398
BG J398
NVPBGJ-398
NVPBGJ 398
NVPBGJ398
BGJ 398
Infigratinib
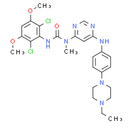
Chemical Name: 3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-(6-((4-(4-ethylpiperazin-1-yl)phenyl)amino)pyrimidin-4-yl)-1-methylurea.
Smiles: CN(C(=O)NC1C(Cl)=C(C=C(OC)C=1Cl)OC)C1=CC(NC2C=CC(=CC=2)N2CCN(CC2)CC)=NC=N1
InChiKey: QADPYRIHXKWUSV-UHFFFAOYSA-N
InChi: InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31)
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO: 1 mg/mLwarmed(1.78 mM).
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
BGJ-398 inhibits FGFR1, FGFR2, and FGFR3 with IC50=~1 nM, FGFR3K650E with IC50=4.9 nM, and FGFR4 with IC50=60 nM. IC50 values for all other kinases are in the μM range (FYN, LCK, YES, and ABL, IC50=1.9, 2.5, 1.1, and 2.3 μM, respectively) except for VEGFR2, KIT, and LYN, which are inhibited at submicromolar concentrations (IC50=0.18, 0.75, and 0.3 μM, respectively). BGJ-398 inhibits the proliferation of the FGFR1-, FGFR2-, and FGFR3-dependent BaF3 cells with IC50 values which are in the low nanomolar range and comparable to those observed for the inhibition of the receptors kinase activity in the enzymatic assay. For the remaining cells, all IC50 values are greater than 1.5 μM except for VEGFR2 (IC50 1449 and 938 nM), for which there is at least a 400-fold selectivity versus FGFR1, FGFR2, and FGFR3. BGJ-398 (ranging between 1 nM and 10 μM) is potent at inhibiting cell growth of FGFR2-mutant endometrial cancer cells.
In Vivo:
BGJ-398 is administered to athymic nude mice implanted subcutaneously with RT112/luc1 tumors: either as a 5 mg/kg intravenous bolus in NMP/PEG200 (1:9, v/v) or orally by gavage as a suspension in PEG300/D5W (2:1, v/v) at a 20 mg/kg dose.The relevant pharmacokinetic (PK) parameters indicate that the oral bioavailability of BGJ-398 in this study is 32%. After intravenous dosing, BGJ-398 shows a rapid distribution from the vascular compartment into the peripheral tissues, translating into a high volume of distribution (26 L/kg). The plasma clearance is high at 3.3 L/h/kg (61% of liver blood flow). The ratio of tumor to plasma after oral dosing based on AUC is determined to be 10. BGJ-398 (30 mg/kg) significantly inhibits the growth of FGFR2-mutated endometrial cancer xenograft models.
References:
- Tran A, Koh TS, Prawira A, Ho RZW, Le TBU, Vu TC, Hartano S, Teo XQ, Chen WC, Lee P, Thng CH, Huynh H. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as Imaging Biomarker for Vascular Normalization Effect of Infigratinib in High- FGFR-Expressing Hepatocellular Carcinoma Xenografts. Mol Imaging Biol. 2020 Sep 9. doi: 10.1007/s11307-020-01531-7. Epub ahead of print. PMID: 32909245.
- Makawita S, K Abou-Alfa G, Roychowdhury S, Sadeghi S, Borbath I, Goyal L, Cohn A, Lamarca A, Oh DY, Macarulla T, T Shroff R, Howland M, Li A, Cho T, Pande A, Javle M. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future Oncol. 2020 Jun 25. doi: 10.2217/fon-2020-0299. Epub ahead of print. PMID: 32580579.
- Magone MT, Hartley IR, Fitzgibbon E, Bishop R, Arango M, Moran S, Vold R, Rivero JD, Pozo K, Streit J, Roszko KL, Collins MT, Gafni RI. Ocular Adverse Effects of Infigratinib, a New Fibroblast Growth Factor Receptor Tyrosine Kinase Inhibitor. Ophthalmology. 2020 Sep 1:S0161-6420(20)30844-7. doi: 10.1016/j.ophtha.2020.08.026. Epub ahead of print. PMID: 32888946.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.