Description
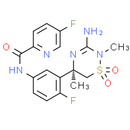
Verubecestat, CAS No. 1286770-55-5, also known as MK-8931 or SCH 900931, is a potent and selective beta-secretase inhibitor, and BACE1 protein inhibitor or Beta-site APP-cleaving enzyme 1 inhibitor. Verubecestat is a promising novel therapeutic drug candidate in Alzheimer's disease. Verubecestat reduced Aβ cerebral spinal fluids (CSF) levels up to 92% and was well tolerated by patients.
Product information
CAS Number: 1286770-55-5
Molecular Weight: 409.41
Formula: C17H17F2N5O3S
Synonym:
MK 8931
MK8931
SCH 900931
SCH900931
MK-8931
MK-8931-009
SCH-900931
Verubecestat free base
Chemical Name: (R)-N-(3-(3-amino-2,5-dimethyl-1,1-dioxido-5,6-dihydro-2H-1,2,4-thiadiazin-5-yl)-4-fluorophenyl)-5-fluoropicolinamide
Smiles: CN1C(N)=N[C@@](C)(CS1(=O)=O)C1=CC(=CC=C1F)NC(=O)C1=CC=C(F)C=N1
InChiKey: YHYKUSGACIYRML-KRWDZBQOSA-N
InChi: InChI=1S/C17H17F2N5O3S/c1-17(9-28(26,27)24(2)16(20)23-17)12-7-11(4-5-13(12)19)22-15(25)14-6-3-10(18)8-21-14/h3-8H,9H2,1-2H3,(H2,20,23)(H,22,25)/t17-/m0/s1
Technical Data
Appearance: Solid Power.
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
How to use
In Vitro:
Verubecestat (MK-8931) is a β-site amyloid precursor protein cleaving enzyme 1/2 (BACE1/2) inhibitor. Verubecestat does not significantly inhibit human CYP isoforms 1A2, 2C9, 2C19, 2D6, and 3A4 (all IC50>40 μM), indicating that the compound is unlikely to be a perpetrator of CYP-mediated drug-drug interactions. Verubecestat has IC50s of 2.1 nM, 0.7 nM, 4.4 nM for Aβ1-40, Aβ1-42, sAPPβ in HEK293 APPSwe/Lon cells.
In Vivo:
Verubecestat (MK-8931; 3 mg/kg; IV or oral) has a T1/2 of 1.9 hours, a CL of 46 mL/min/kg, a Vss of 5.4 L/kg, a C max of 0.27 μM and a AUC of 1.1 μM•h for Sprague-Dawley (SD) rats. Verubecestat (1 mg/kg; IV) has a T1/2 of 4.9 hours, a CL of 21 mL/min/kg, a Vss of 7.5 L/kg for cynomolgus monkeys. Verubecestat (1 mg/kg; IV) has a T1/2 of 9.7 hours, a CL of 4.3 mL/min/kg, a Vss of 2.7 L/kg for beagle dogs. Verubecestat (30 mg/kg; orally; BID for 5 days) causes a modest (1.4-fold) induction of CYP 3A1 activity but does not significantly alter the expression of CYPs 1A1, 1A2, 2B, 3A2, or 4A in rats. Verubecestat dose-dependently reduces CSF and cortex Aβ40 with ED50 values of 5 and 8 mg/kg, respectively, corresponding to unbound plasma EC50 values of 48 and 81 nM, respectively. Verubecestat (3 and 10 mg/kg; orally) reduces profound, sustained of CSF Aβ40 levels and has peak effects on CSF Aβ lowering (72 and 81% reduction at 3 and 10 mg/kg, respectively) 12 h after dosing.
References:
- Thaisrivongs DA, Morris WJ, Tan L, Song ZJ, Lyons TW, Waldman JH, Naber JR, Chen W, Chen L, Zhang B, Yang J. A Next Generation Synthesis of BACE1 Inhibitor Verubecestat (MK-8931). Org Lett. 2018 Mar 16;20(6):1568-1571. doi: 10.1021/acs.orglett.8b00259. Epub 2018 Feb 26. PubMed PMID: 29481097.
- Chen W, Meng D, N'Zemba B, Morris WJ. Palladium-Catalyzed Enantioselective Synthesis of Cyclic Sulfamidates and Application to a Synthesis of Verubecestat. Org Lett. 2018 Mar 2;20(5):1265-1268. doi: 10.1021/acs.orglett.7b03639. Epub 2018 Feb 20. PubMed PMID: 29461065.
- Barhate CL, Lopez DA, Makarov AA, Bu X, Morris WJ, Lekhal A, Hartman R, Armstrong DW, Regalado EL. Macrocyclic glycopeptide chiral selectors bonded to core-shell particles enables enantiopurity analysis of the entire verubecestat synthetic route. J Chromatogr A. 2018 Mar 2;1539:87-92. doi: 10.1016/j.chroma.2018.01.042. Epub 2018 Jan 31. PubMed PMID: 29397980.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.