Description
Bexarotene, CAS No. 153559-49-0, also known as LGD-1069, is a synthetic retinoic acid agent with potential antineoplastic, chemopreventive, teratogenic and embryotoxic properties. Bexarotene selectively binds to and activates retinoid X receptors (RXRs), thereby inducing changes in gene expression that lead to cell differentiation, decreased cell proliferation, apoptosis of some cancer cell types, and tumor regression.
Product information
CAS Number: 153559-49-0
Molecular Weight: 348.48
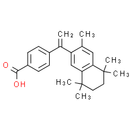
Formula: C24H28O2
Synonym:
LGD1069
LGD 1069
LG 100069
SR 11247
3-methyl TTNEB
Bexarotene
Targretin
LGD-1069
Ro 26-445
Chemical Name: 4-(1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)vinyl)benzoic acid
Smiles: CC1=CC2=C(C=C1C(=C)C1C=CC(=CC=1)C(O)=O)C(C)(C)CCC2(C)C
InChiKey: NAVMQTYZDKMPEU-UHFFFAOYSA-N
InChi: InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26)
Technical Data
Appearance: Solid Power.
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: Soluble in DMSO, not in water
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined.
HS Tariff Code: 382200
References:
- Qu L, Tang X. Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol. 2010 Jan;65(2):201-5. doi: 10.1007/s00280-009-1140-4. Epub 2009 Sep 24. Review. PubMed PMID: 19777233.
- Gniadecki R, Assaf C, Bagot M, Dummer R, Duvic M, Knobler R, Ranki A, Schwandt P, Whittaker S. The optimal use of bexarotene in cutaneous T-cell lymphoma. Br J Dermatol. 2007 Sep;157(3):433-40. Epub 2007 Jun 6. Review. PubMed PMID: 17553039.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.