Description
Acadesine, also known as AICA-riboside, and AICAR, is an AMP-activated protein kinase activator which is used for the treatment of acute lymphoblastic leukemia and may have applications in treating other disorders such as diabetes. Acadesine is an adenosine regulating agent developed by PeriCor Therapeutics and licensed to Schering-Plough in 2007 for phase III studies. The drug is a potential first-in-class agent for prevention of reperfusion injury in CABG surgery. Schering began patient enrollment in phase III studies in May, 2009.
Product information
CAS Number: 2627-69-2
Molecular Weight: 258.23
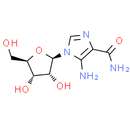
Formula: C9H14N4O5
Synonym:
ARA100
ARA-100
ARA 100
GP 1 110
SCH-900395
SCH 900395
SCH900395
AICA ribofuranoside
AICA ribonucleoside
AICA riboside
AICAR
Chemical Name: 5-amino-1-[(2R, 3R, 4S, 5R)-3, 4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]imidazole-4-carboxamide
Smiles: NC1=C(N=CN1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C(N)=O
InChiKey: RTRQQBHATOEIAF-UUOKFMHZSA-N
InChi: InChI=1S/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO: 51 mg/mL(197.49 mM).
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
Acadesine (500 μM) increases the ZMP content in extracts of isolated hepatocytes after up to 30-40 min treatment, then remains fairly constant at approximately 4 nmol/g. Acadesine (500 μM) causes a transient 12-fold activation of AMPK at 15 min in rat hepatocytes and 2-3 fold activation of AMPK in adipocytes, without affecting levels of ATP, ADP or AMP. Acadesine (500 μM) causes a dramatic inhibition of both fatty acid and sterol synthesis in rat hepatocytes. Acadesine (500 μM) also causes a dramatic inactivation of HMG-CoA reductase. Acadesine induces apoptosis of B-CLL cells in a dose-dependent manner with EC50 of 380 μM. Acadesine (0.5 mM) decreases cell viability of B-CLL cells from 20 representative patients from 68% to 26%. Acadesine (0.5 mM) induces caspase activation and cytochrome crelease from mitochondria. Uptake and phosphorylation of Acadesine (0.5 mM) are required to induce apoptosis and activate AMPK in B-CLL cells. Acadesine (2-4 mM) only slightly affects the viability of T cells from B-CLL patients, Acadesine (0.5 mM) remarkedly reduces viability of B cells but not T cells. Acadesine triggers loss of cell metabolism in K562, LAMA-84 and JURL-MK1 and is also effective in killing imatinib-resistant K562 cells and Ba/F3 cells carrying the T315I-BCR-ABL mutation. The effect of Acadesine is abrogated by GF109203X and Ro-32-0432, both inhibitor of classical and new PKCs and accordingly, Acadesine triggers relocation and activation of several PKC isoforms in K562 cells. Acadesine dose-dependently inhibits K562 colony formation at day 10, the growth inhibitory effect of acadesine is already detected at 0.25 mM and is maximal at 2.5 mM. Acadesine causes a concentration-related reduction in CD18 expression on LPS-stimulated neutrophils in vitro. Acadesine significantly (1 mM) inhibits N-formyl-methionyl-leucyl-phenylalanine-induced granulocyte CD11b up-regulation by a mean of 61% in blood.
In Vivo:
Acadesine (50 mg/kg) significantly reduces tumor formation in a mouse xenograft model of K562 cells. Acadesine (10 mg/kg) results in higher fluid required to stabilize hemodynamics in pigs. Acadesine (10 mg/kg) inhibits LPS-induced protein permeability of pulmonary capillaries, peak inspiratory pressures on constant tidal volume and dead space ventilation in pigs.
References:
- Robert G, et al. PLoS One, 2009, 4(11), e7889.
- Campàs C, et al. Blood, 2003, 101(9), 3674-368
- Corton JM, et al. Eur J Biochem, 1995, 229(2), 558-565.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.