Description
Zosuquidar (LY335979) is an inhibitor of P-glycoprotein with a Ki value of 59 nM.
Product information
CAS Number: 167354-41-8
Molecular Weight: 527.60
Formula: C32H31F2N3O2
Related CAS Number:
167465-36-3 (Zosuquidar trihydrochloride)
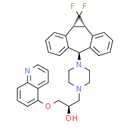
Chemical Name: (2R)-1-{4-[(2R, 4S, 11S)-3, 3-difluorotetracyclo[10.4.0.0, .0, ]hexadeca-1(12), 5, 7, 9, 13, 15-hexaen-11-yl]piperazin-1-yl}-3-(quinolin-5-yloxy)propan-2-ol
Smiles: O[C@H](CN1CCN(CC1)[C@@H]1C2=CC=CC=C2[C@@H]2[C@H](C3=CC=CC=C31)C2(F)F)COC1=CC=CC2=NC=CC=C21
InChiKey: IHOVFYSQUDPMCN-DBEBIPAYSA-N
InChi: InChI=1S/C32H31F2N3O2/c33-32(34)29-22-7-1-3-9-24(22)31(25-10-4-2-8-23(25)30(29)32)37-17-15-36(16-18-37)19-21(38)20-39-28-13-5-12-27-26(28)11-6-14-35-27/h1-14,21,29-31,38H,15-20H2/t21-,29-,30+,31-/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO : 8.33 mg/mL (15.79 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
References:
- Dantzig AH, et al. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996 Sep 15;56(18):4171-9.
- Marcelletti JF, Multani PS, Lancet JE et al. Leukemic blast and natural killer cell P-glycoprotein function and inhibition in a clinical trial of zosuquidar infusion in acute myeloid leukemia. Leuk Res. 2009 Jun;33(6):769-74.
- Ruff P, Vorobiof DA, Jordaan JP et al. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother Pharmacol. 2009 Sep;64(4):763-8.
- Cripe LD, Uno H, Paietta EM et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood. 2010 Nov 18;116(20):4077-85.
- Abu Ajaj K, Graeser R, Kratz F. Zosuquidar and an albumin-binding prodrug of zosuquidar reverse multidrug resistance in breast cancer cells of doxorubicin and an albumin-binding prodrug of doxorubicin. Breast Cancer Res Treat. 2012 Jul;134(1):117-29.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.