Description
Satraplatin, also known as JM216 and BMS182751, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues—cisplatin, carboplatin, and oxaliplatin—must be given intravenously.
Product information
CAS Number: 129580-63-8
Molecular Weight: 500.28
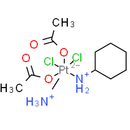
Formula: C10H22Cl2N2O4Pt
Synonym:
BMS182751
BMY45594
JM216
POplat
Chemical Name: Satraplatin
Smiles: CC(=O)O[Pt-2]([NH3+])(Cl)(Cl)([NH2+]C1CCCCC1)OC(C)=O
InChiKey: CKNPWBAXEKSCRG-UHFFFAOYSA-J
InChi: InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+4/p-4
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.