Description
Avibactam is a non-β-lactam β-lactamase inhibitor antibiotic, which is a new drug application for avibactam in combination with ceftazidime, and was approved by the FDA on February 25, 2015, for treating complicated urinary tract and complicated intra-abdominal Infections caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant gram-negative bacterial pathogens.
Product information
CAS Number: 1192500-31-4
Molecular Weight: 265.24
Formula: C7H11N3O6S
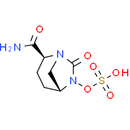
Chemical Name: [(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl]oxidanesulfonic acid
Smiles: NC(=O)[C@@H]1CC[C@@H]2CN1C(=O)N2OS(O)(=O)=O
InChiKey: NDCUAPJVLWFHHB-UHNVWZDZSA-N
InChi: InChI=1S/C7H11N3O6S/c8-6(11)5-2-1-4-3-9(5)7(12)10(4)16-17(13,14)15/h4-5H,1-3H2,(H2,8,11)(H,13,14,15)/t4-,5+/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.