Description
LDE225 is a potent and specific Hedgehog pathway inhibitor through binding and antagonizing Smo with an IC50 of 11 nM. It is currently in the clinical trials to treat cancers. It inhibited Hh signaling and induces tumor regression in animal models of medulloblastoma. It sensitized chemotherapy-resistant ovarian cancer cell lines to paclitaxel, but not to carboplatin. In one of the clinical trials, treatment with 0.75% LDE225 cream in NBCCS patients was very well tolerated and caused BCC regression.
Product information
CAS Number: 956697-53-3
Molecular Weight: 485.50
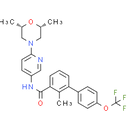
Formula: C26H26F3N3O3
Synonym:
LDE225
LDE 225
LDE-225
NVP-LDE225
NVP-LDE-225
NVP LDE225
Erismodegib
Sonidegib
Odomzo
Chemical Name: N-(6-((2S,6R)-2,6-dimethylmorpholino)pyridin-3-yl)-2-methyl-4'-(trifluoromethoxy)biphenyl-3-carboxamide
Smiles: CC1C(=CC=CC=1C(=O)NC1=CN=C(C=C1)N1C[C@H](C)O[C@H](C)C1)C1=CC=C(C=C1)OC(F)(F)F
InChiKey: VZZJRYRQSPEMTK-CALCHBBNSA-N
InChi: InChI=1S/C26H26F3N3O3/c1-16-14-32(15-17(2)34-16)24-12-9-20(13-30-24)31-25(33)23-6-4-5-22(18(23)3)19-7-10-21(11-8-19)35-26(27,28)29/h4-13,16-17H,14-15H2,1-3H3,(H,31,33)/t16-,17+
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO up to 100 mM
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
LDE225 was used at 1 µM final concentration in cellular assays.
In Vivo:
LDE225 was dosed orally at 20 mg/kg once per day or 10 mg/kg twice per day (formulation: PEG300/5% dextrose in water 75:25 v/v)
References:
- Pan S, et al. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. (2010) ACS Med. Chem. Lett., 1 (3), pp 130–134.
- Buonamici S, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. (2010) Sci Transl Med. 2(51):51ra70.
- Skvara H, et al. Topical treatment of Basal cell carcinomas in nevoid Basal cell carcinoma syndrome with a smoothened inhibitor. (2011) J Invest Dermatol. 131(8):1735-44.
- Steg AD, et al. Smoothened Antagonists Reverse Taxane Resistance in Ovarian Cancer. Mol Cancer Ther. 2012 in press.
- Heller E, et al. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. (2012) Cancer Res. 72(4):897-907.
Products are for research use only. Not for human use.
Documents
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.