Description
AT6 is a PROTAC AT1 analogue, which is a highly selective bromodomain (Brd4) degrader.
Product information
CAS Number: 2098836-50-9
Molecular Weight: 1004.68
Formula: C48H58ClN9O7S3
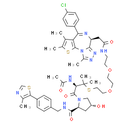
Chemical Name: (2S, 4R)-1-[(2R)-3-({2-[2-(2-{2-[(9S)-7-(4-chlorophenyl)-4, 5, 13-trimethyl-3-thia-1, 8, 11, 12-tetraazatricyclo[8.3.0.0, ]trideca-2(6), 4, 7, 10, 12-pentaen-9-yl]acetamido}ethoxy)ethoxy]ethyl}sulfanyl)-2-acetamido-3-methylbutanoyl]-4-hydroxy-N-{[4-(4-methyl-1, 3-thiazol-5-yl)phenyl]methyl}pyrrolidine-2-carboxamide
Smiles: CC(=O)N[C@H](C(=O)N1C[C@H](O)C[C@H]1C(=O)NCC1C=CC(=CC=1)C1SC=NC=1C)C(C)(C)SCCOCCOCCNC(=O)C[C@@H]1N=C(C2=C(SC(C)=C2C)N2C1=NN=C2C)C1C=CC(Cl)=CC=1
InChiKey: ZCEIHPCVOJGWHG-TZPPCSJFSA-N
InChi: InChI=1S/C48H58ClN9O7S3/c1-27-29(3)68-47-40(27)41(33-12-14-35(49)15-13-33)54-37(44-56-55-30(4)58(44)47)23-39(61)50-16-17-64-18-19-65-20-21-67-48(6,7)43(53-31(5)59)46(63)57-25-36(60)22-38(57)45(62)51-24-32-8-10-34(11-9-32)42-28(2)52-26-66-42/h8-15,26,36-38,43,60H,16-25H2,1-7H3,(H,50,61)(H,51,62)(H,53,59)/t36-,37+,38+,43-/m1/s1
Technical Data
Appearance: Solid Power
Purity: ≥98% (or refer to the Certificate of Analysis)
Solubility: DMSO : 100 mg/mL (99.53 mM; Need ultrasonic)
Shipping Condition: Shipped under ambient temperature as non-hazardous chemical or refer to Certificate of Analysis
Storage Condition: Dry, dark and -20 oC for 1 year or refer to the Certificate of Analysis.
Shelf Life: ≥12 months if stored properly.
Stock Solution Storage: 0 - 4 oC for 1 month or refer to the Certificate of Analysis.
Drug Formulation: To be determined
HS Tariff Code: 382200
How to use
In Vitro:
PROTACs (proteolysis-targeting chimaeras) are bifunctional molecules that recruit a target protein in proximity to an E3 ubiquitin ligase to trigger protein degradation. Structural elucidation of the key ternary ligase: PROTAC: target species and how this impacts target degradation selectivity remains elusive. The ligand folds into itself to allow formation of specific intermolecular interactions in the ternary complex. Isothermal titration calorimetry studies, supported by surface mutagenesis and proximity assays, are consistent with pronounced cooperative formation of ternary complexes with Brd4BD2. Structure-based-designed compound AT1 exhibits highly selective depletion of Brd4 in cells.
References:
- Gadd MS, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017 May;13(5):514-521.
Products are for research use only. Not for human use.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.